which hydrocarbon is unsaturated A simple introduction to organic chemistry
Hey there, fellow science enthusiasts! Today we’re going to delve into the fascinating world of organic chemistry and explore the concept of unsaturated hydrocarbons. Let’s get started!
If you’re new to organic chemistry, it can seem quite daunting at first. But don’t worry, we’ll start with the basics and build up from there. So, what exactly are unsaturated hydrocarbons?
Understanding Unsaturated Hydrocarbons
Hydrocarbons, as the name suggests, are molecules that contain carbon and hydrogen atoms. They are the building blocks of organic compounds and can be found in a wide range of natural and synthetic materials, such as petroleum, plastics, and even living organisms.
When it comes to hydrocarbons, there are two main categories: saturated and unsaturated. Saturated hydrocarbons are molecules that contain only single bonds between the carbon atoms, which means that they are “saturated” with hydrogen atoms. Examples of saturated hydrocarbons include methane, ethane, and propane.
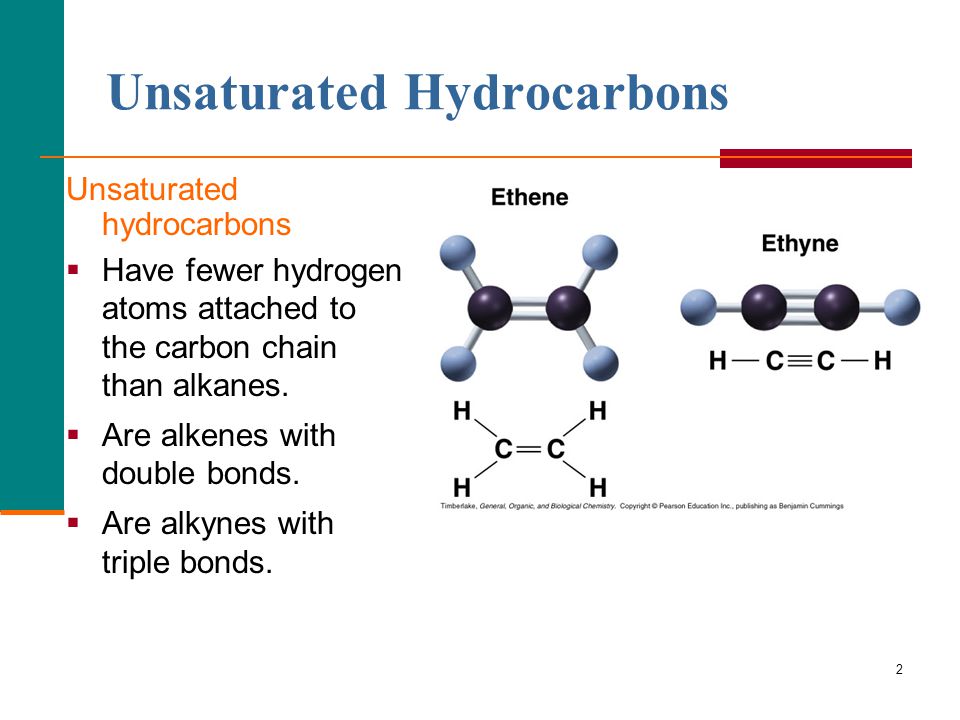
On the other hand, unsaturated hydrocarbons are molecules that contain at least one double or triple bond between the carbon atoms. These bonds can create unique properties that make unsaturated hydrocarbons useful in a variety of applications.
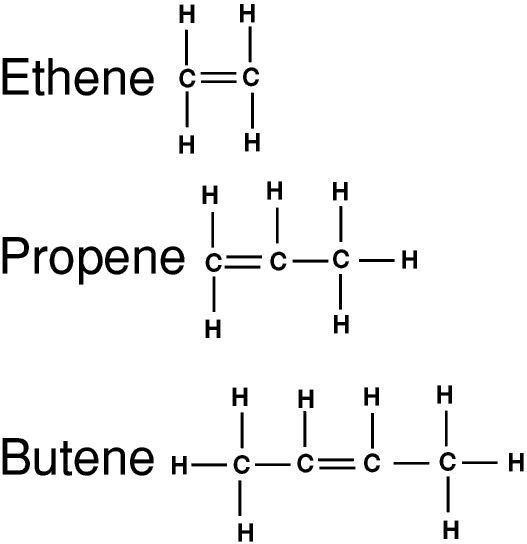 The Role of Alkenes
The Role of Alkenes
One of the most important types of unsaturated hydrocarbons is alkenes. Alkenes are molecules that contain a double bond between the carbon atoms. This double bond gives alkenes unique properties, such as the ability to undergo addition reactions and form polymers.
Alkenes are important in many industries, from being used as solvents in the production of pharmaceuticals to being used as feedstocks in the production of plastics. Some common examples of alkenes include ethene (also known as ethylene), propene (also known as propylene), and butene.
 Other Unsaturated Hydrocarbons
Other Unsaturated Hydrocarbons
Alkenes aren’t the only type of unsaturated hydrocarbons, though. Other examples include alkynes (which contain a triple bond between the carbon atoms), arenes (which contain an aromatic ring), and dienes (which contain two double bonds).
Each of these types of unsaturated hydrocarbons has its own unique properties and applications. For example, alkynes are often used in the production of rubber and plastics, while arenes are important components of many industrial chemicals and drugs.
So there you have it – a brief introduction to the world of unsaturated hydrocarbons. Hopefully, this has given you a better understanding of what they are and why they’re important. Until next time, keep exploring the amazing world of science!
If you are looking for PPT - Ch. 11: Unsaturated Hydrocarbons PowerPoint Presentation, free you’ve visit to the right web. We have 5 Images about PPT - Ch. 11: Unsaturated Hydrocarbons PowerPoint Presentation, free like PPT - What are unsaturated hydrocarbons? PowerPoint Presentation, free, SS2 Chemistry Third Term: Unsaturated Hydrocarbons- Alkynes | Passnownow and also A simple introduction to organic chemistry. Read more:
PPT - Ch. 11: Unsaturated Hydrocarbons PowerPoint Presentation, Free
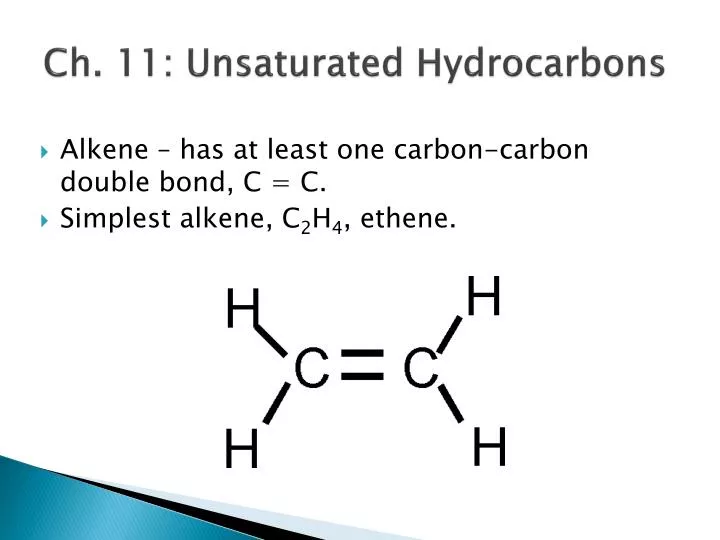 www.slideserve.comunsaturated hydrocarbons
www.slideserve.comunsaturated hydrocarbons
PPT - What Are Unsaturated Hydrocarbons? PowerPoint Presentation, Free
 www.slideserve.comunsaturated hydrocarbons ppt saturated carbon powerpoint presentation skip video atoms alkanes hydrogen
www.slideserve.comunsaturated hydrocarbons ppt saturated carbon powerpoint presentation skip video atoms alkanes hydrogen
Unsaturated Hydrocarbon: Definition & Examples - Video & Lesson
 education-portal.comexamples unsaturated hydrocarbon hydrocarbons alkynes uses definition structures common study aromatic unlock
education-portal.comexamples unsaturated hydrocarbon hydrocarbons alkynes uses definition structures common study aromatic unlock
A Simple Introduction To Organic Chemistry
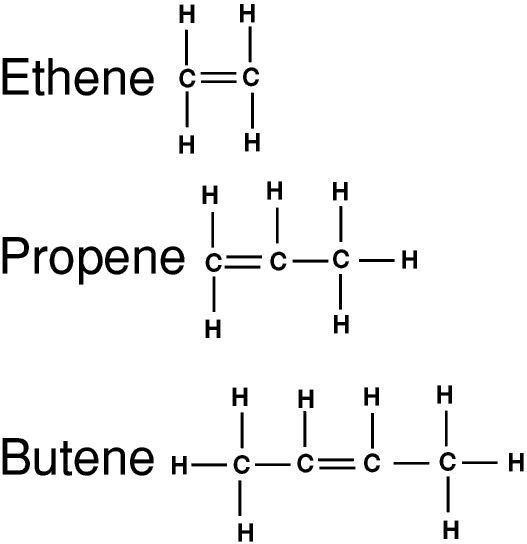 barrygraygillingham.comalkene alkenes hydrocarbons alkanes types chemistry carbon bond double different alkynes alkyne molecule hydrocarbon organic unsaturated simple single aliphatic bonds
barrygraygillingham.comalkene alkenes hydrocarbons alkanes types chemistry carbon bond double different alkynes alkyne molecule hydrocarbon organic unsaturated simple single aliphatic bonds
SS2 Chemistry Third Term: Unsaturated Hydrocarbons- Alkynes | Passnownow
 passnownow.comunsaturated hydrocarbons chemistry passnownow
passnownow.comunsaturated hydrocarbons chemistry passnownow
Unsaturated hydrocarbons chemistry passnownow. Alkene alkenes hydrocarbons alkanes types chemistry carbon bond double different alkynes alkyne molecule hydrocarbon organic unsaturated simple single aliphatic bonds. Unsaturated hydrocarbon: definition & examples